Magnesium And Potassium Nitrate Equation . Potassium + magnesium nitrate = potassium nitrate + magnesium. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the. The calcium ion and the nitrate ion; K + mg(no3)2 = kno3 + mg is a single. If you're behind a web filter,. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. if you're seeing this message, it means we're having trouble loading external resources on our website. The potassium ion and the sulfate ion; write the chemical formula for an ionic compound composed of each pair of ions. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−.
from www.numerade.com
kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. If you're behind a web filter,. Potassium + magnesium nitrate = potassium nitrate + magnesium. The potassium ion and the sulfate ion; Predict which forms an anion, which forms a cation, and the. The calcium ion and the nitrate ion; mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. magnesium and nitrogen react to form an ionic compound. if you're seeing this message, it means we're having trouble loading external resources on our website.
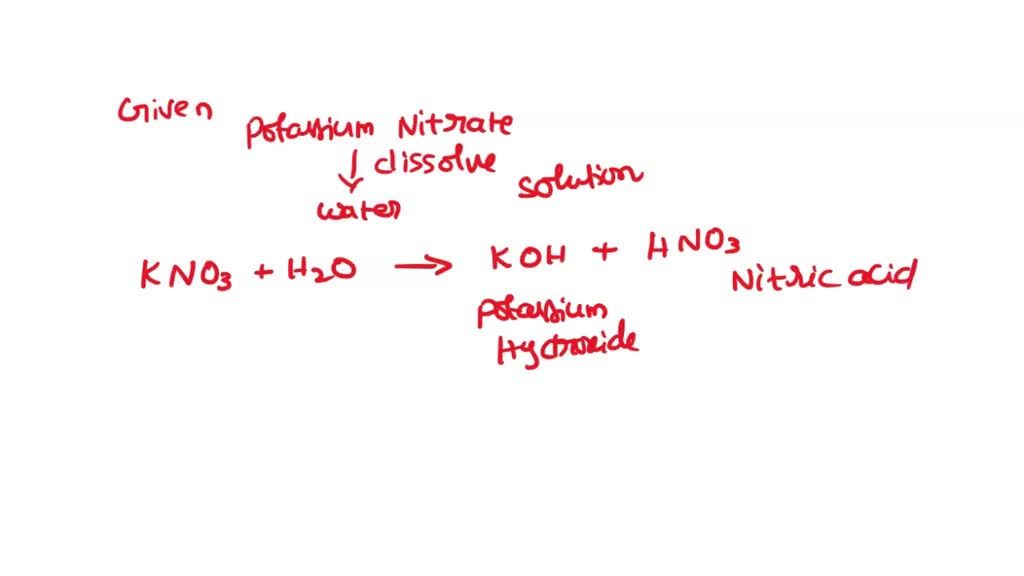
SOLVED 'QUESTION 9 potassium nitrate, KNO3 dissolves water, H2O, the most significant
Magnesium And Potassium Nitrate Equation K + mg(no3)2 = kno3 + mg is a single. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. If you're behind a web filter,. The calcium ion and the nitrate ion; mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. if you're seeing this message, it means we're having trouble loading external resources on our website. Predict which forms an anion, which forms a cation, and the. write the chemical formula for an ionic compound composed of each pair of ions. The potassium ion and the sulfate ion; magnesium and nitrogen react to form an ionic compound. K + mg(no3)2 = kno3 + mg is a single. Potassium + magnesium nitrate = potassium nitrate + magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−.
From www.youtube.com
Equation for NaNO3 + H2O (Sodium nitrate + Water) YouTube Magnesium And Potassium Nitrate Equation if you're seeing this message, it means we're having trouble loading external resources on our website. Potassium + magnesium nitrate = potassium nitrate + magnesium. K + mg(no3)2 = kno3 + mg is a single. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. The potassium ion and. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVED1) Please balance the following equations A) Ag2SO4 + BaCI2 > AgCI + BaSO4 B) Pb Magnesium And Potassium Nitrate Equation Predict which forms an anion, which forms a cation, and the. K + mg(no3)2 = kno3 + mg is a single. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. if you're seeing this message, it means we're having trouble loading external resources on our website. Potassium + magnesium nitrate = potassium nitrate. Magnesium And Potassium Nitrate Equation.
From www.shutterstock.com
57 Magnesium Nitrate Formula Images, Stock Photos & Vectors Shutterstock Magnesium And Potassium Nitrate Equation If you're behind a web filter,. Potassium + magnesium nitrate = potassium nitrate + magnesium. The potassium ion and the sulfate ion; if you're seeing this message, it means we're having trouble loading external resources on our website. Predict which forms an anion, which forms a cation, and the. K + mg(no3)2 = kno3 + mg is a single.. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVED 'er on test tube hydroxide and magnesium nitrate were mixed ervations when potassium Magnesium And Potassium Nitrate Equation The potassium ion and the sulfate ion; Potassium + magnesium nitrate = potassium nitrate + magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. mg (no3)2 + koh = mg (oh)2 +. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVEDWrite the net ionic equation for the reaction between solutions of (a) Aluminum nitrate Magnesium And Potassium Nitrate Equation write the chemical formula for an ionic compound composed of each pair of ions. If you're behind a web filter,. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. if you're seeing this message, it means we're having trouble loading external resources on our website. mg (no3)2 + koh = mg. Magnesium And Potassium Nitrate Equation.
From www.youtube.com
IGCSE Chemistry lesson 34 part c Thermal of nitrates YouTube Magnesium And Potassium Nitrate Equation If you're behind a web filter,. magnesium and nitrogen react to form an ionic compound. K + mg(no3)2 = kno3 + mg is a single. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. The potassium ion and the sulfate ion; Predict which forms an anion, which forms a cation, and the. . Magnesium And Potassium Nitrate Equation.
From www.slideshare.net
Action of heat on chemical compound e book Magnesium And Potassium Nitrate Equation magnesium and nitrogen react to form an ionic compound. The calcium ion and the nitrate ion; Predict which forms an anion, which forms a cation, and the. write the chemical formula for an ionic compound composed of each pair of ions. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. K +. Magnesium And Potassium Nitrate Equation.
From dxogmqfsy.blob.core.windows.net
Magnesium Nitrate Plus Potassium Hydroxide Formula at Stefanie Hales blog Magnesium And Potassium Nitrate Equation magnesium and nitrogen react to form an ionic compound. Potassium + magnesium nitrate = potassium nitrate + magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. kno3 +. Magnesium And Potassium Nitrate Equation.
From www.pw.live
Potassium Nitrate Formula, Structure, Properties, Uses Magnesium And Potassium Nitrate Equation Potassium + magnesium nitrate = potassium nitrate + magnesium. magnesium and nitrogen react to form an ionic compound. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. The calcium ion and the nitrate ion; K + mg(no3)2 = kno3 + mg is a single. mg (no3)2 +. Magnesium And Potassium Nitrate Equation.
From www.shutterstock.com
Potassium Nitrate Formula Handwritten Chemical Formula Stock Illustration 1707498511 Shutterstock Magnesium And Potassium Nitrate Equation if you're seeing this message, it means we're having trouble loading external resources on our website. K + mg(no3)2 = kno3 + mg is a single. magnesium and nitrogen react to form an ionic compound. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. write the. Magnesium And Potassium Nitrate Equation.
From www.chegg.com
Solved 1. Write out the formula for magnesium nitrate and Magnesium And Potassium Nitrate Equation mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. The calcium ion and the nitrate ion; Potassium + magnesium nitrate = potassium nitrate + magnesium. if you're seeing this message, it means we're having trouble loading external resources on our website. The potassium ion and the. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVEDWhich acid and base react to give an aqueous solution of magnesium nitrate? Write a Magnesium And Potassium Nitrate Equation The calcium ion and the nitrate ion; magnesium and nitrogen react to form an ionic compound. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. The potassium ion and the sulfate ion; mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous. Magnesium And Potassium Nitrate Equation.
From www.youtube.com
Writing the Formula for Potassium Nitrate YouTube Magnesium And Potassium Nitrate Equation magnesium and nitrogen react to form an ionic compound. If you're behind a web filter,. mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. The potassium ion and the sulfate ion;. Magnesium And Potassium Nitrate Equation.
From socratic.org
What occurs when magnesium nitrate is mixed with potassium sulfate in aqueous solution? Socratic Magnesium And Potassium Nitrate Equation write the chemical formula for an ionic compound composed of each pair of ions. The potassium ion and the sulfate ion; mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. K + mg(no3)2 = kno3 + mg is a single. Predict which forms an anion, which. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVED The following molecular equation represents the reaction that occurs when aqueous Magnesium And Potassium Nitrate Equation If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. The potassium ion and the sulfate ion; write the chemical formula for an ionic compound composed of each pair of ions. Predict which forms an anion, which forms a cation, and the. mg (no3)2 +. Magnesium And Potassium Nitrate Equation.
From slideplayer.com
Quantitative Chemistry ppt download Magnesium And Potassium Nitrate Equation If you're behind a web filter,. mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous magnesium. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. K + mg(no3)2 = kno3 + mg is a single. The potassium ion and the sulfate ion;. Magnesium And Potassium Nitrate Equation.
From www.numerade.com
SOLVED The video shows the addition of aqueous sodium carbonate to a solution of aqueous Magnesium And Potassium Nitrate Equation The calcium ion and the nitrate ion; K + mg(no3)2 = kno3 + mg is a single. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2−. kno3 + mgso4 = mg(no3)2 + k2so4 is a double displacement (metathesis) reaction where two moles of aqueous potassium. if you're seeing this message, it means. Magnesium And Potassium Nitrate Equation.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium And Potassium Nitrate Equation Potassium + magnesium nitrate = potassium nitrate + magnesium. K + mg(no3)2 = kno3 + mg is a single. if you're seeing this message, it means we're having trouble loading external resources on our website. The potassium ion and the sulfate ion; mg (no3)2 + koh = mg (oh)2 + kno3 is a double displacement (metathesis) reaction where. Magnesium And Potassium Nitrate Equation.